American Association for Laboratory Accreditation (A2LA) is among the largest accreditation bodies in the world and the only independent, 501(c)3, non-profit, internationally recognized accreditation body in the United States that offers a full range of comprehensive conformity assessment accreditation services.
Established in 1978 as a public service membership society, A2LA is dedicated to the formal recognition of competent testing and calibration laboratories (including medical laboratories), biobanking facilities, inspection bodies, product certification bodies, proficiency testing providers, and reference material producers. A2LA has over 4,300 actively accredited certificates representing all 50 US states and more than 50 countries. For a current listing of A2LA’s accredited organizations, please search our directory.
A2LA offers three primary service lines to support recognition of competence in conformity assessment industries.
Accreditation
Accreditation is synonymous with both the quality and competence of an organization, based on international standard(s). Accreditation refers to the recognition given to an organization by an authoritative body such as A2LA. It is a process by which an authoritative body gives formal recognition that a Conformity Assessment Body (CAB) fulfills specified requirements and is competent to carry out specific tasks.
Accreditation is the most appropriate way to ensure an organization’s competence in performing a specified task. By encompassing the human element of the CAB, end-users can be sure that their data is accurate and dependable. A2LA is a third-party body that performs accreditations to various international standards, ensuring an unbiased and objective evaluation of your organization’s functions. A2LA provides a range of accreditation programs using international standards, including:
- ISO/IEC 17025 for Testing/Calibration Laboratories
- ISO/IEC 17020 for Inspection Bodies
- ISO/IEC 17043 for Proficiency Testing Providers
- ISO/IEC 17065 for Product Certification Bodies
- ISO/IEC 17029 Validation and Verification
- ISO 15189 and CLIA for Clinical Testing Laboratories
- ISO 17034 for Reference Material Producers
- ISO 20387 for Biobanking Facilities
Annual Conference
The A2LA Annual Conference (formerly known as Tech Forum) has grown to become one of the largest, multidiscipline events in the accreditation industry, attracting attendees from over a dozen different industries, including automotive, environmental, pharmaceutical, and calibration. The forum promotes discussion and training on topics related to the accreditation industry through face-to-face interactions with staff, members, assessors, accredited organizations, and other interested parties. Join a global group of industry professionals for:
- Accreditation program-specific technical sessions
- Training and educational sessions
- Professional development soft skill sessions
- Networking
Membership
Membership with A2LA empowers individuals and organizations to participate in the development of new accreditation standards, network with others working in the quality industry, gain valuable knowledge and training in new industries, and support and promote quality and public safety. Our members include private organizations, public institutions, and academic institutions of all sizes. A2LA membership and accreditation are separate programs.
Although membership is not a requirement for accreditation, we strongly encourage all A2LA-accredited organizations to become members to have a stronger voice in the organization and receive additional membership benefits.
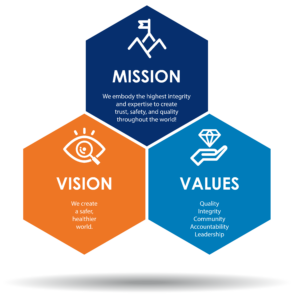
Mission
We embody the highest integrity and expertise to create trust, safety, and quality throughout the world.
Vision
We create a safer, healthier world.
Core Values
A2LA’s core values were developed collectively by our staff to reflect our culture, objectives, and priorities. We work to exemplify these five values in every aspect of our activities, whether internal or external:
- Quality: Striving for Excellence
- Integrity: Maintaining Trust Through Honoring Our Commitments
- Community: Customer Focused, Service Driven, People First
- Accountability: Set, Share, and Exceed Expectations, Make it Happen
- Leadership: Advocating for a Better World Through Accreditation
Governance
A2LA is governed by a Board of Directors.
Organizations are assessed by trained Assessors who are technical experts in their fields.
Accreditation decisions are made by the Accreditation Council.
The day-to-day operation of the organization is handled by the Staff Members at A2LA Headquarters in Frederick, MD.
A2LA Accreditation Agreements
To ensure the acceptance of work performed by A2LA-accredited organizations, A2LA has sought recognition and approval from international, domestic, and industry groups. Our accreditation programs are internationally-recognized and based on globally-accepted criteria resulting in A2LA’s signatory status with the following international recognition arrangements:
- International Laboratory Accreditation Cooperation (ILAC) Mutual Recognition Arrangement (MRA)
- International Accreditation Forum (IAF) Multilateral Recognition Arrangement (MLA)
- Asia Pacific Accreditation Cooperation (APAC) Mutual Recognition Arrangement (MRA)
A2LA’s domestic recognitions include Federal, State, Local, and industry partner confirmation that A2LA’s programs meet the highest standards for quality. References to our recognitions can be found here.
A2LA’s Commitment to Impartial Services
Consistent with the principles set forth in national and international standards, it is required that A2LA’s services be impartial and objective, free from any undue commercial, financial and other pressures that could compromise impartiality, and uninfluenced by the private interests of individuals acting for or on behalf of A2LA. A2LA invites interested parties to submit potential risks of impartiality relating to its accreditation activities through its contact us feature located at the bottom of all A2LA web pages.
